forum
lZenxl's Honesty Queue <Mania> ( CLOSED )
posted
Total Posts
574
Mod Rating !
Total votes: 59
Topic Starter
nold_1702 wrote:
What square root of 25?
>>5
http://osu.ppy.sh/s/157896
smoke weed everyday
mania+taiko mod thank you
hue hue hue wrong :3
i think i know the anwer , but i have no map for this round >w<
Topic Starter
ha noNwolf wrote:
-5
http://osu.ppy.sh/s/160313
do I win nao
2 more slot y u no have perfect solution
I will leave this queue open until tommorow then I will check o/
Answer the Previous previous post Question
lZenxl wrote:
OKAY OPEN 2 SLOTS SO I CAN MOD TOMMOROW
Trick Question: What square root of 25?
The answer is not as simple as you think, well it might be for asians, so...
I win don't I ? D:
I mean, I have stopped learning maths two years ago ;-;
I need help with hitsounds on my map, could you check please ? (Link in sig, length ~2:00)
Topic Starter
Answer is indeed (+/-) 5TaMul wrote:
±5
http://osu.ppy.sh/s/135017
thanks
There are 2 answers for this question :3
PyaKura wrote:
I win don't I ? D:
I mean, I have stopped learning maths two years ago ;-;
I need help with hitsounds on my map, could you check please ? (Link in sig, length ~2:00)
wow okay seems legit
best photoshop evar.
Will mod all 4 maps even though I "rejected" them
Cause I'm a very bored person
CLOSE CLOSED
Topic Starter
OPEN DIS QUHUHUE FOR 2 Slots for Tommorowzors
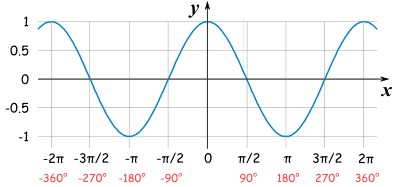
Question : What is cos(90°) ?
The answer is 0 heh just started learning trigonometry huehuehue
Taiko mod pls~
https://osu.ppy.sh/s/158380
Taiko mod pls~
https://osu.ppy.sh/s/158380
Topic Starter
CORRECTO
Will mod Tommorowzors
Will mod Tommorowzors
Zenx-san why is your schoolwork spilling into your osu owo
Topic Starter
CoroQuetz wrote:
Zenx-san why is your schoolwork spilling into your osu owo
I'm addicted to math haha... ha... *runs*
Organic chemistry next please.
Topic Starter
Okay get ready to chemister WHEWneonat wrote:
Organic chemistry next please.
Topic Starter
It's closed, sorry :cShikiNoHollow wrote:
https://osu.ppy.sh/s/160162
4k/7k
mod please
SYNC.ART'S - Road to Departure
SB checking reqs~
if the SB Checking Queue is open
btw
this Queue is closed
SB checking reqs~
if the SB Checking Queue is open
btw
this Queue is closed
Topic Starter
OPEN FOR 2 SLOT M4M ( All of my sub-queues are open )
Mainly Taiko OR Standard, if you can find any errors in mania I will be happy to change it
Link : https://osu.ppy.sh/s/132824
Mod at least 1 diff., I will be happy if you can mod more than that, it's okay if you are not experienced in modding, another person's opinion may be an inspiration to others.
Mainly Taiko OR Standard, if you can find any errors in mania I will be happy to change it
Link : https://osu.ppy.sh/s/132824
Mod at least 1 diff., I will be happy if you can mod more than that, it's okay if you are not experienced in modding, another person's opinion may be an inspiration to others.
Topic Starter
Acceptooo I will mod on Tuesday though, I'm a bit busy on Monday :c0gre wrote:
https://osu.ppy.sh/forum/p/2985705#p2985705
M4M on taiko map
is it ok ?
https://osu.ppy.sh/s/110126
oni
muzukashii
1 More M4M Slot ( Read previous Post! )
Topic Starter
AHA NO HOMEWORK
Open for 2 NM Slots for ANYTHING
1. You are ONLY allowed to answer 1 Question
2. If the person before you got the first question correct, you are not allowed to answer it again
3. If you don't know the answer, just paint some jibberish : http://puu.sh/7NTSJ.jpg
Open for 2 NM Slots for ANYTHING
Extra Rules :neonat wrote:
Organic chemistry next please.
1. You are ONLY allowed to answer 1 Question
2. If the person before you got the first question correct, you are not allowed to answer it again
3. If you don't know the answer, just paint some jibberish : http://puu.sh/7NTSJ.jpg
Answer these Questions:
Question 1 : What is the Enthalpy of Combustion Equation for Carbon?
1. CO(g) + O(g) -> CO2(g)
2. C(l) + O2(g) -> CO2(g)
3. 2C(s) + 2O2(g) -> 2CO2(g)
4. C(s) + O2(g) -> CO2(g)
Question 2 : Why does 1 mol of NaCl ( Sodium Chlroide ) weigh more than 1 mol of O3 ( Ozone )? Explain.
Question 1 : What is the Enthalpy of Combustion Equation for Carbon?
1. CO(g) + O(g) -> CO2(g)
2. C(l) + O2(g) -> CO2(g)
3. 2C(s) + 2O2(g) -> 2CO2(g)
4. C(s) + O2(g) -> CO2(g)
Question 2 : Why does 1 mol of NaCl ( Sodium Chlroide ) weigh more than 1 mol of O3 ( Ozone )? Explain.
Question 2's answer:
...Good luck to myself?
If I'm somehow correct, can I get a ticket? lol
EDIT: Now no one will believe me if I say that I took the social science program in high school \:D/ /runs
Na's mass is 22.99, while Cl's is 35.45. If you add them, the value will be 58.44 grams/mole. Meanwhile, oxygen's mass is 16, and O3 means there are three atoms of oxygen (did I use the right term lol), so 16 * 3 = 48 grams/mole.
Since salt (NaCl)'s molar mass (58.44 grams/mole) is higher than ozone (O3)'s (48)... Well, I've already answered it, but I'll repeat it again to emphasize: Salt has a higher molar mass value than ozone (since one mole of salt equals to 58.44 grams of it, from what "grams/mole" implies, and ozone only has 48).
Since salt (NaCl)'s molar mass (58.44 grams/mole) is higher than ozone (O3)'s (48)... Well, I've already answered it, but I'll repeat it again to emphasize: Salt has a higher molar mass value than ozone (since one mole of salt equals to 58.44 grams of it, from what "grams/mole" implies, and ozone only has 48).
...Good luck to myself?
If I'm somehow correct, can I get a ticket? lol
EDIT: Now no one will believe me if I say that I took the social science program in high school \:D/ /runs
Topic Starter
CORRECTO, now I realise how the ticket system works, will add a ticket-sectionHinsvar wrote:
Question 2's answer:Na's mass is 22.99, while Cl's is 35.45. If you add them, the value will be 58.44 grams/mole. Meanwhile, oxygen's mass is 16, and O3 means there are three atoms of oxygen (did I use the right term lol), so 16 * 3 = 48 grams/mole.
Since salt (NaCl)'s molar mass (58.44 grams/mole) is higher than ozone (O3)'s (48)... Well, I've already answered it, but I'll repeat it again to emphasize: Salt has a higher molar mass value than ozone (since one mole of salt equals to 58.44 grams of it, from what "grams/mole" implies, and ozone only has 48).
...Good luck to myself?
If I'm somehow correct, can I get a ticket? lol
READ MY PREVIOUS POST
mother of rules o.o
Topic Starter
rule-okasanReikosaka wrote:
mother of rules o.o
lZenxl wrote:
AHA NO HOMEWORK
Open for 2 NM Slots for ANYTHINGExtra Rules :neonat wrote:
Organic chemistry next please.
1. You are ONLY allowed to answer 1 Question
2. If the person before you got the first question correct, you are not allowed to answer it again
3. If you don't know the answer, just paint some jibberish : http://puu.sh/7NTSJ.jpgAnswer these Questions:
Question 1 : What is the Enthalpy of Combustion Equation for Carbon?
1. CO(g) + O(g) -> CO2(g)
2. C(l) + O2(g) -> CO2(g)
3. 2C(s) + 2O2(g) -> 2CO2(g)
4. C(s) + O2(g) -> CO2(g)
Question 2 : Why does 1 mol of NaCl ( Sodium Chlroide ) weigh more than 1 mol of O3 ( Ozone )? Explain.
Question 1:
1:2C(s)+O2(g) -> 2CO(g)
2:2CO(g)+O2(g) -> 2CO2(g)
I believe this is the answer >_>
Topic Starter
wait wat, why is there 2 answers, it's a MCQ ( Multiple choice question ), I will allow you to guess one more time if you wantexamination wrote:
lZenxl wrote:
AHA NO HOMEWORK
Open for 2 NM Slots for ANYTHING
Extra Rules :
1. You are ONLY allowed to answer 1 Question
2. If the person before you got the first question correct, you are not allowed to answer it again
3. If you don't know the answer, just paint some jibberish : http://puu.sh/7NTSJ.jpgAnswer these Questions:
Question 1 : What is the Enthalpy of Combustion Equation for Carbon?
1. CO(g) + O(g) -> CO2(g)
2. C(l) + O2(g) -> CO2(g)
3. 2C(s) + 2O2(g) -> 2CO2(g)
4. C(s) + O2(g) -> CO2(g)
Question 2 : Why does 1 mol of NaCl ( Sodium Chlroide ) weigh more than 1 mol of O3 ( Ozone )? Explain.
Question 1:
1:2C(s)+O2(g) -> 2CO(g)
2:2CO(g)+O2(g) -> 2CO2(g)
I believe this is the answer >_>
QUESTION 2 HAS BEEN ANSWERED
3. 2C(s) + 2O2(g) -> 2CO2(g)
No no,do you see wat I'm writing? The Combustion Equation for Carbon actually contains 2 procedures.lZenxl wrote:
wait wat, why is there 2 answers, it's a MCQ ( Multiple choice question ), I will allow you to guess one more time if you wantexamination wrote:
Question 1:
1:2C(s)+O2(g) -> 2CO(g)
2:2CO(g)+O2(g) -> 2CO2(g)
I believe this is the answer >_>
QUESTION 2 HAS BEEN ANSWERED
So if you want me to choose only one,it will be 3
Topic Starter
No no,do you see wat I'm writing? The Combustion Equation for Carbon actually contains 2 procedures.lZenxl wrote:
wait wat, why is there 2 answers, it's a MCQ ( Multiple choice question ), I will allow you to guess one more time if you wantexamination wrote:
Question 1:
1:2C(s)+O2(g) -> 2CO(g)
2:2CO(g)+O2(g) -> 2CO2(g)
I believe this is the answer >_>
QUESTION 2 HAS BEEN ANSWERED
So if you want me to choose only one,it will be 3[/quote]
Orz, I didn't realise you split them up in 2 parts, anyways, it's technically still wrong ( because you wrote down the enthalpy of combustion of 2C ) but I'll accept it anyways since it's a minor mistake
Added ticket to both!Fycho wrote:
3. 2C(s) + 2O2(g) -> 2CO2(g)
SPOILER
THE ANSWER IS ACTUALLY 4 , but I'm kind
REASONING :
The enthalpy of combustion is where 1 mol of substance ( in this case Carbon ) is burnt in excess Oxygen (O2 GAS)
Therefore, the stoichiometry of Carbon IS always 1
C(solid) + O2(gas) -> CO2(gas)
oh since you say there's no maps hmm...lZenxl wrote:
CLOSEDDDDDDReopening in 1 hour, since there's no maps...
https://osu.ppy.sh/s/158376 take a look~
2 taiko diffs need mods

_(:3」∠)_Well my high school chemistry teacher says burning works like that,and should be split into 2 equations ;w;
Topic Starter
XD it is indeed 2 equations but the enthalpy of combustion equation is always 1 step.examination wrote:
oh since you say there's no maps hmm...lZenxl wrote:
CLOSEDDDDDDReopening in 1 hour, since there's no maps...
https://osu.ppy.sh/s/158376 take a look~
2 taiko diffs need mods
_(:3」∠)_Well my high school chemistry teacher says burning works like that,and should be split into 2 equations ;w;
The more detailed equation for ALL enthalpy of combustion equations:
Where x,y,z are stoichiometric numbers and A is the substance burnt
1A + xO2 -> yCO2 + zH2O
Will mod the map tommorow!
STILL CLOSED I HAVE TO DO STUFF will reopen in like 30 minutes
Topic Starter
Woops fell alseep
OPEN 1 SLOT
OPEN 1 SLOT
(Math) Question : What is the derivative of a constant?
The derivative of a constant, that is to say, a term that does not contain a variable, is zero.
http://osu.ppy.sh/s/123760
much thanks (tired of seeing this map yet? Haha)~
raika's looking for taiko mods, so just my taikos will be sufficient~
http://osu.ppy.sh/s/123760
much thanks (tired of seeing this map yet? Haha)~
raika's looking for taiko mods, so just my taikos will be sufficient~
Topic Starter
ACCEPTOCoroQuetz wrote:
The derivative of a constant, that is to say, a term that does not contain a variable, is zero.
http://osu.ppy.sh/s/123760
much thanks (tired of seeing this map yet? Haha)~
raika's looking for taiko mods, so just my taikos will be sufficient~
Will mod tommorow
SPOILER
I won't get tired of CQ's kat rhythms <3
CLOSED CLOSED
Topic Starter
OKAYS OPEN FOR 2 NM
Form a sentence/a paragraph that consists of 10 "a"s and 10 "c"s, no more no less
It doesn't have to make sense, but the words must be found in the english dictionary
Form a sentence/a paragraph that consists of 10 "a"s and 10 "c"s, no more no less
It doesn't have to make sense, but the words must be found in the english dictionary